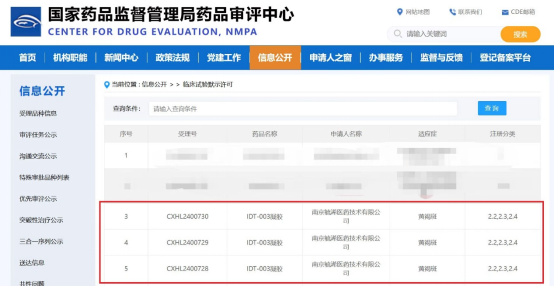
Indetek Lab recently announced that its self-developed new drug, IDT-003 Gel, has officially entered the Phase II clinical study and has successfully completed the enrollment of the first subject. This Phase II clinical study, led by Professor Wu Liming, Director of the Dermatology Department at Hangzhou First People's Hospital, is a multicenter, randomized, open-label, blinded evaluation, positive-controlled parallel-group clinical trial. It aims to systematically evaluate the efficacy and safety of IDT-003 Gel in patients with moderate to severe melasma.
IDT-003 Gel is developed based on Indetek Lab's proprietary ILDS®; transdermal delivery system. This technology efficiently promotes the targeted delivery of active ingredients to the epidermal basal layer and dermis, significantly increasing the effective concentration and retention time of the drug in skin tissues, thereby optimizing therapeutic efficacy. Simultaneously, the ILDS®; system can reduce the proportion of drug absorption into the bloodstream through the skin, minimizing systemic exposure risks and further ensuring medication safety. In terms of user experience, IDT-003 Gel employs a once-daily dosing regimen, offering convenient application and effectively improving patient compliance. Its refreshing, transparent gel base provides a comfortable feel upon application and is particularly suitable for long-term facial use, precisely meeting patients' core need for "balancing efficacy and aesthetics."
Previously completed Phase I clinical trials have confirmed the favorable safety and tolerability profile of IDT-003 Gel in humans. Preclinical research data indicates that the gel demonstrates significantly superior efficacy and safety compared to the positive control drug in both natural pigmentation models and melasma animal models.
The initiation of the Phase II clinical study for IDT-003 Gel marks a new milestone in the drug's development, paving a new path for exploring innovative treatment options for moderate to severe melasma.